Surgical dressing trade mark IGAR Lightpore type (spunlace base)
Surgical dressing trade mark IGAR Lightpore type (spunlace base) is made on the basis of high-quality spunlace material, which allows skin to breathe of white colour.
These surgical dressings are very popular because they are high quality, there is a wide range of sizes, they are well attached and easy to remove. Dressings have well absorbing pad.
Аdvantage:
- Surgical dressings are made of high quality material - spunlace
- Hypoallergenic and elastic, keep skin “breathing”
- Wide selection of sizes
- Sterile
- Removed easily and without pain
Specifications:
- The base is high-quality, breathable spunlace material, white colour.
- Adhesive – hypoallergenic glue.
- Surgical dressings have edges that are firmly fixed and not peeled off.
- Elastic and tight to break.
- Reliable absorbing pad.
- The adhesive side of the dressing is protected by glossy paper.
- Sterile dressings, available in individual packing.
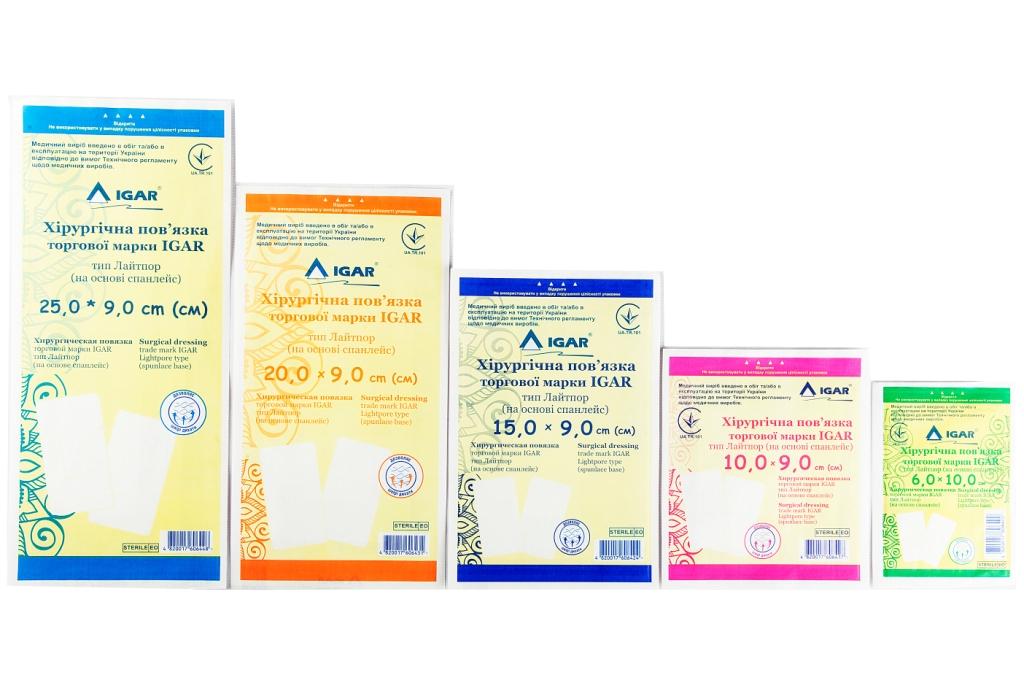
The range of IGAR surgical dressings of various sizes:
Surgical dressing trade mark IGAR Lightpore type (spunlace base) 25,0 × 9,0 сm
Surgical dressing trade mark IGAR Lightpore type (spunlace base)20,0 × 9,0 сm
Surgical dressing trade mark IGAR Lightpore type (spunlace base)15,0 × 9,0 сm
Surgical dressing trade mark IGAR Lightpore type (spunlace base) 10,0 × 9,0 сm
Surgical dressing trade mark IGAR Lightpore type (spunlace base) 6,0 × 10,0 сm
Size/Packing/Morion Code:
Packaging: 1 piece in individual paper packing.
| Proportions | Packing rate, /pair/ | Morion Code | |
|---|---|---|---|
| group | transport | ||
| 25,0 × 9,0 сm | 50 | 600 | 229989 |
| 20,0 × 9,0 сm | 50 | 1200 | 229988 |
| 15,0 × 9,0 сm | 50 | 1200 | 229991 |
| 10,0 × 9,0 сm | 50 | 1200 | 229992 |
| 6,0 × 10,0 сm | 50 | 1200 | 227200 |
Shelf life:
Shelf life: 5 years.
Certification:
The medical device is declared and placed on the market of Ukraine according to the requirements of Technical regulations and Conformity Assessment of medical devices, the product has a complete package of documents and packing labelling in Ukrainian language according to the national standards and Technical regulations.
Quality management system of the Manufacturer meets the requirements of DSTU EN ISO 13485
Declaration of Conformity №19-03 / 2016-IGAR