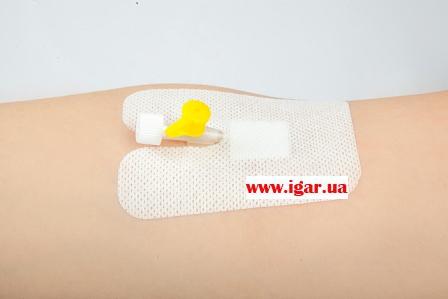
Bactericidal plaster trade mark IGAR Lightpore type (spunlace base) 8,0 × 6,0 cm for IV catheter fixation
Bactericidal plaster trade mark IGAR Lightpore type (spunlace base) 8,0 × 6,0 cm for IV catheter fixation, the base material is spunlace, white colour.
It is U-shaped and designed for fixation of an intravenous catheter.
Sterile and easy to remove.
Аdvantages:
- Special plaster for fixation of an intravenous catheter
- Sterile and convenient to use
- Guaranteed tight fixation of the catheter
- Easy to remove
Specifications:
- base – spunlace, breathable
- adhesive – hypoallergenic adhesive
- there is a special cutout for fixation of the intravenous catheter
Proportion/Packing/Morion Code:
Packaging: 1 piece in an individual package, 100 pieces in a group package / 2,400 pieces in a transport box.
| Proportion | Packing rate, /piece/ | Morion Code | |
|---|---|---|---|
| group | transport | ||
| 8,0 × 6,0 сm | 100 | 2 400 | 227203 |
Application:
bactericidal, sterile plaster is intended for fixation of an intravenous catheter.
Shelf life:
Shelf life: 3 years
Manufacturer: “IGAR Ltd”,Ukraine
Certification:
The medical device is declared and placed on the market of Ukraine according to the requirements of Technical regulations and Conformity Assessment of medical devices, the product has a complete package of documents and packing labelling in Ukrainian language according to the national standards and Technical regulations.
Quality management system of the Manufacturer meets the requirements of DSTU EN ISO 13485.
Содержимое вкладки “Инструкция”
Содержимое вкладки “Сертификаты”
Содержимое вкладки “Декларации”