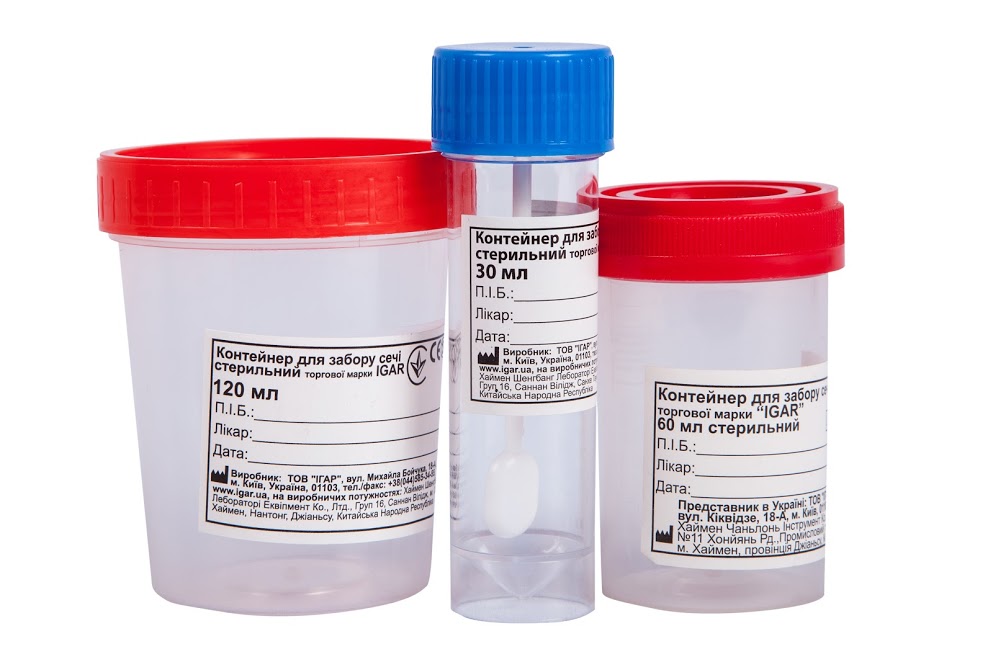
Biological liquids collection containers sterile trade mark IGAR
Biological liquids collection containers sterile trade mark IGAR are very popular among our customers. They are made of medical polystyrene with a tight, screw cap, for safe transportation of biomaterials. IGAR containers are sterile (each container is individually wrapped), have transparent walls, with a graduated scale, and a paper label for signature.
The assortment of the company “IGAR” Ltd presents containers for the collection of biological liquid with volumes of 30, 60, 120 ml.
Аdvantages:
- High-quality containers are made of medical polystyrene
- The containers are convenient, transparent. There are tight caps, scale labelling, labels for signature
- The containers are sterile. Each container is individually wrapped
- There is an assortment of different sizes
Specifications:
- Biological liquids collection containers are made from medical polystyrene with a high density polyethylene cover.
- At room temperature the containers are chemically resistant to all commonly used reagents.
- There are transparent walls, graduated scale and tight screw caps.
- The containers are supplied with a paper label for signatures.
- Each container is packed in an individual plastic bag.
- The containers are sterilized with ethylene oxide.
Sizes/Packing/Morion Code:
Package: 1 piece in an individual plastic bag/100-(400) 500 pieces in a transport package.
| Sizes | Packing rate, /pieces/ | Morion Code: | |
|---|---|---|---|
| group | transport | ||
| 30 ml | 100 | 500 | 240495 |
| 60 ml | 100 | 500 | 240502 |
| 120 ml | 100 | 400 | 240499 |
Application:
Containers IGAR are designed to collect, transport and store samples of biological materials, including urine and fecals.
In addition, our clients told us that IGAR containers are widely used in other areas. They are popular with artists (for storing paints); hand-made by masters (for storing small elements for decoration, paints); in everyday life (for storage of small items: screws, nails, etc.).
Shelf life:
Shelf life: 3 years
Certification:
The medical device is declared and placed on the market of Ukraine according to the requirements of Technical regulations and Conformity Assessment of medical devices, the product has a complete package of documents and packing labelling in Ukrainian language according to the national standards and Technical regulations.
Quality management system of the Manufacturer meets the requirements of DSTU EN ISO 13485.
Declaration of Conformity №15-12/2016-IGAR