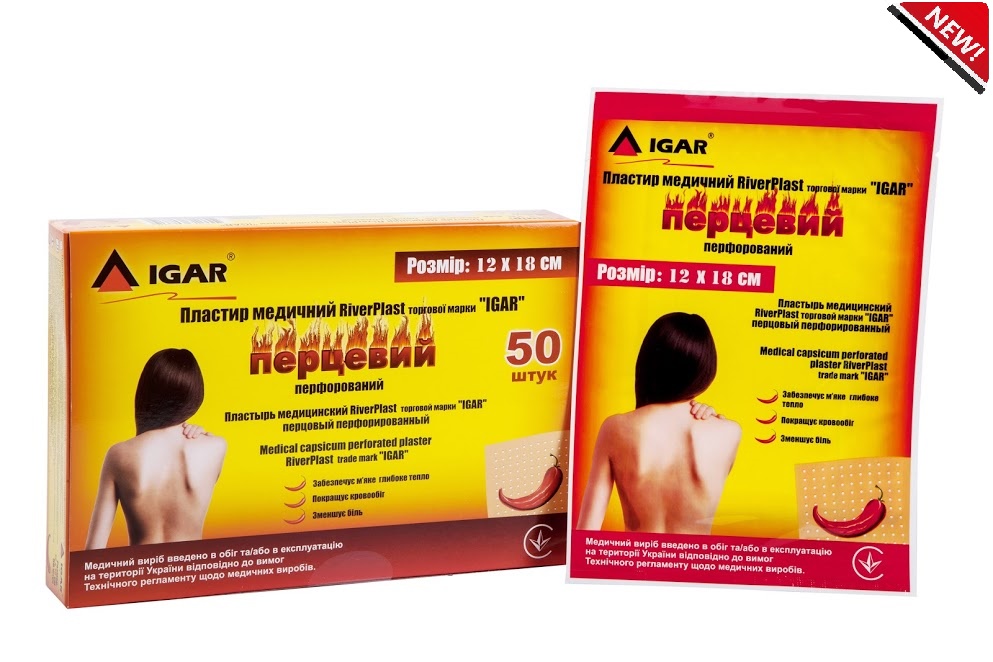
Medical capsicum perforated plaster RiverPLAST trade mark “IGAR” 12 х 18 cm
Medical capsicum perforated plaster RiverPLAST trade mark “IGAR” 12 х 18 cm – has a thin cotton base, which is uniformly сovered with adhesive component, with active substances.
Capsicum plaster trade mark “IGAR” has anesthetic and irritant effect.
It enhances blood circulation, reduces pain, provides soft deep heat.
Аdvantages:
- Capsicum plaster has a perforation that allows skin to breathe
- High quality glue base
- Optimal size and high quality
- Does not leave marks on the skin after removal
Specifications:
- Active ingredients – capsicum extract 2.89%; camphor 9.6%; menthol 7.8%
- Size: 12 x 18 cm
- The plster has a perforation that allows skin to breathe
- High-quality adhesive base, does not leave marks on the skin after removal.
Size/Packing/Morion Code:
Packaging: 1 piece in an individual cardboard package, 50 pieces in a group package / 600 pieces in a transport package.
Size |
Packing rate, /pieces/ | Morion Code | |
|---|---|---|---|
| group | transport | ||
| 12 × 18 cm | 50 | 600 | 478392 |
Application:
Indications: as a local irritant and analgesic reflexogenic means for pain syndromes of various etiologies. In particular, it can be used in complex therapy: radiculitis, myositis, lumbago, arthralgia, osteochondrosis and mononeuritis. The action consists in distracting from pain in the focus of inflammation, as well as in mild muscle relaxant and point effects. In neurology, this tool is used in the treatment of painful muscle spasm, myositis. The use of this tool is wide spread in the treatment of colds, cough, rhinitis, inflammatory processes in the joints and other diseases .
Way of application. Outwardly. Before applying the plaster skin must be degreased, then dried. Take out the protective film from the plaster and put it on the skin, gently press it and smooth it. The plaster is intended for single use only. It can not be removed during the day, if there is no strong irritation. In case of strong burning sensation, the plaster must be removed and the skin needs to be smeared with cream. If there are traces of adhesive mass left on the skn, they can be removed with a soap solution.
Side effect. Allergic reactions, burning in the place of application are possible. Avoid contact with eyes and mucous surfaces. Clean and dry hands after using the medical device.
Contraindications. Hypersensitivity to the active components. Do not use on the open wounds, birthmarks, moles, tumors, burns.
Shelf life:
Shelf life: 5 years
Storage conditions: Keep away from children, avoid excessively high temperatures, excessive air humidity and direct sunlight.
Manufacturer: “IGAR Ltd”,Ukraine
Certification:
The medical device is declared and placed on the market of Ukraine according to the requirements of Technical regulations and Conformity Assessment of medical devices, the product has a complete package of documents and packing labelling in Ukrainian language according to the national standards and Technical regulations.
Quality management system of the Manufacturer meets the requirements of DSTU EN ISO 13485
Declaration of Conformity №7-08 / 2016 -IGAR
Содержимое вкладки “Инструкция”
Содержимое вкладки “Сертификаты”
Содержимое вкладки “Декларации”